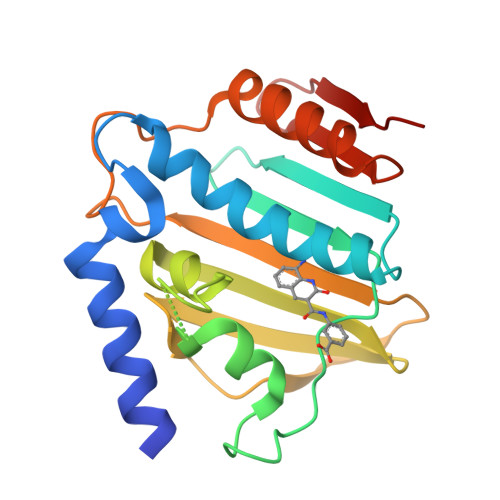
Lead Identification of 8-(Methylamino)-2-oxo-1,2-dihydroquinoline Derivatives as DNA Gyrase Inhibitors: Hit-to-Lead Generation Involving Thermodynamic Evaluation.
Ushiyama, F., Amada, H., Takeuchi, T., Tanaka-Yamamoto, N., Kanazawa, H., Nakano, K., Mima, M., Masuko, A., Takata, I., Hitaka, K., Iwamoto, K., Sugiyama, H., Ohtake, N.(2020) ACS Omega 5: 10145-10159
- PubMed: 32391502
- DOI: https://doi.org/10.1021/acsomega.0c00865
- Primary Citation of Related Structures:
6KZV, 6KZX, 6KZZ, 6L01 - PubMed Abstract:
DNA gyrase and topoisomerase IV are well-validated pharmacological targets, and quinolone antibacterial drugs are marketed as their representative inhibitors. However, in recent years, resistance to these existing drugs has become a problem, and new chemical classes of antibiotics that can combat resistant strains of bacteria are strongly needed. In this study, we applied our hit-to-lead (H2L) chemistry for the identification of a new chemical class of GyrB/ParE inhibitors by efficient use of thermodynamic parameters. Investigation of the core fragments obtained by fragmentation of high-throughput screening hit compounds and subsequent expansion of the hit fragment was performed using isothermal titration calorimetry (ITC). The 8-(methylamino)-2-oxo-1,2-dihydroquinoline derivative 13e showed potent activity against Escherichia coli DNA gyrase with an IC 50 value of 0.0017 μM. In this study, we demonstrated the use of ITC for primary fragment screening, followed by structural optimization to obtain lead compounds, which advanced into further optimization for creating novel antibacterial agents.
Organizational Affiliation:
Chemistry Laboratories, Taisho Pharmaceutical Company Ltd., 1-403, Yoshino-Cho, Kita-Ku, Saitama, Saitama 331-9530, Japan.